Neisseria meningitidis
From Wikipedia, the free encyclopedia
|
|
This article needs attention from an expert on the subject. (May 2011) |
| Neisseria meningitidis | |
|---|---|
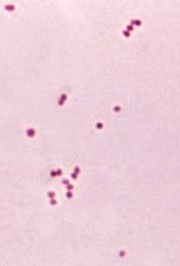 |
|
| Photomicrograph of N. meningitidis | |
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Betaproteobacteria |
| Order: | Neisseriales |
| Family: | Neisseriaceae |
| Genus: | Neisseria |
| Species: | N. meningitidis |
| Binomial name | |
| Neisseria meningitidis Albrecht & Ghon 1901 |
|
Contents
History
In 1884 Ettore Marchiafava and Angelo Celli first observed the bacterium inside cells in the cerebral spinal fluid (CSF).[1] In 1887 Anton Weichselbaum isolated the bacterium from the CSF of patients with bacterial meningitis.[2] He named the bacterium Diplococcus intracellularis meningitidis.[1]Epidemiology
N. meningitidis is a major cause of illness, developmental impairment and death during childhood in industrialized countries and has been responsible for epidemics in Africa and in Asia. Every year, about 2,500 to 3,500 people become infected with N. meningitidis in the US, with a frequency of about 1 in 100,000. Children younger than 5 years are at greatest risk, followed by teenagers of high school age. Rates in the African meningitis belt were as high as 1 in 1,000 to 1 in 100[3] before introduction of a vaccine in 2010. The incidence of meningococcal disease is highest among infants (children younger than 1-year-old) whose immune system is relatively immature. In industrialized countries there is a second peak of incidence in young adults, who are congregating closely, living in dormitories or smoking.[4]It is spread through saliva and other respiratory secretions during coughing, sneezing, kissing, and chewing on toys. Inhalation of respiratory droplets from a carrier which may be someone who is themselves in the early stages of disease can transmit the bacteria. Close contact with a carrier is the predominant risk factor. Other risk factors include a weakened general or local immune response, such as a recent upper respiratory infection, smoking, and complement deficiency. The incubation period is short, from 2 to 10 days. In susceptible individuals, N. meningitidis may invade the bloodstream and cause a systemic infection, sepsis, disseminated intravascular coagulation, breakdown of circulation, and septic shock.
Microbiology
N. meningitidis is a Gram-negative bacterium since it has an outer and inner membranes with a thin layer of peptidoglycan in between. It tests positive for the enzyme cytochrome c oxidase.[5]Habitat
N. meningitidis is a part of the normal nonpathogenic flora in the nasopharynx of up to 5–15% of adults.[6] It colonizes and infects only humans, and has never been isolated from other animals; this is thought to stem from the bacterium's inability to get iron other than from the human sources transferrin and lactoferrin.[7]Subtypes
Disease-causing strains are classified according to the antigenic structure of their polysaccharide capsule.[citation needed] Serotype distribution varies markedly around the world.[8] Among the 13 identified capsular types of N. meningitidis, six (A, B, C, W135, X, and Y) account for most disease cases worldwide.[9] Type A has been the most prevalent in Africa and Asia, but is rare/ practically absent in North America. In the United States, serogroup B is the predominant cause of disease and mortality, followed by serogroup C. The multiple subtypes have hindered development of a universal vaccine for meningococcal disease.Virulence
Lipooligosaccharide (LOS) is a component of the outer membrane of N. meningitidis. This acts as an endotoxin and is responsible for septic shock and hemorrhage due to the destruction of red blood cells.[10] Other virulence factors include a polysaccharide capsule which prevents host phagocytosis and aids in evasion of the host immune response; fimbriae mediate attachment of the bacterium to the epithelial cells of the nasopharynx.[11][12] It infects the cell by sticking to it mainly with long thin extensions called pili and the surface-exposed proteins Opa and Opc.[13] Meningococci produce an IgA protease, an enzyme that cleaves IgA class antibodies and thus allows the bacteria to evade a subclass of the humoral immune system.A hypervirulent strain was discovered in China. Its impact is yet to be determined.[3]
Clinical laboratory identification
With a fatality risk approaching 15% within 12 hours of infection, it is crucial to initiate testing as quickly as possible, but not to wait for the results before initiating antibiotic therapy.A small amount of cerebrospinal fluid (CSF) is sent to the laboratory as soon as possible for analysis. The diagnosis is suspected, when gram negative diplococci are seen on Gram stain of a centrifuged sample of CSF; sometimes they are located inside white blood cells. The microscopic identification takes around 1–2 hours after specimen arrival in the laboratory.[14]
The gold standard of diagnosis is microbiological isolation of N. meningitidis by growth from a sterile body fluid, which could be CSF or blood.[3] Diagnosis is confirmed when the organism has grown, most often on a chocolate agar plate. To differentiate any bacterial growth from other species a small amount of a bacterial colony is tested for oxidase, catalase for which all clinically relevant Neisseria show a positive reaction, and the carbohydrates maltose, sucrose, and glucose, in which N. meningitidis will ferment that is, utilize the glucose and maltose. Finally, serology determines the subgroup of the N. meningitidis, which is important for epidemiological surveillance purposes; this may often only be done in specialized laboratories.
The above tests take a minimum of 48–72 hours turnaround time for growing the organism, and up to a week more for serotyping. Growth can and often does fail, either because antibiotics have been given preemptively, or because specimens have been inappropriately transported, as the organism is extremely susceptible to antibiotics and fastidious in its temperature, CO2 and growth medium requirements.
Polymerase chain reaction tests where available, mostly in industrialized countries, have been increasingly used; PCR can rapidly identify the organism, and works even after antibiotics have been given.[3]
Signs and symptoms
Main article: Meningococcal disease
Meningococcus can cause meningitis and other forms of meningococcal disease.[14] It initially produces general symptoms like fatigue, fever, and headache and can rapidly progress to neck stiffness,
coma and death in 10% of cases. Symptoms of meningococcal meningitis
are easily confused with those caused by other bacteria, such as Hemophilus influenzae and Streptococcus pneumoniae.[7] [3] Suspicion of meningitis is a medical emergency
and immediate medical assessment is recommended. Current guidance in
the United Kingdom is that if a case of meningococcal meningitis or septicaemia (infection of the blood) is suspected intravenous antibiotics should be given and the ill person admitted to the hospital.[15] This means that laboratory tests may be less likely to confirm the presence of Neisseria meningitidis
as the antibiotics will dramatically lower the number of bacteria in
the body. The UK guidance is based on the idea that the reduced ability
to identify the bacteria is outweighed by reduced chance of death.Septicaemia caused by Neisseria meningitidis has received much less public attention than meningococcal meningitis even though septicaemia has been linked to infant deaths.[16] Meningococcal septicaemia typically causes a purpuric rash, that does not lose its color when pressed with a glass ("non-blanching") and does not cause the classical symptoms of meningitis. This means the condition may be ignored by those not aware of the significance of the rash. Septicaemia carries an approximate 50% mortality rate over a few hours from initial onset. Many health organizations advise anyone with a non-blanching rash to go to a hospital as soon as possible.[citation needed] Not all cases of a purpura-like rash are due to meningococcal septicaemia, but other possible causes need prompt investigation as well (e.g. ITP a platelet disorder or Henoch-Schönlein purpura).
Other severe complications include Waterhouse-Friderichsen syndrome, a massive, usually bilateral, hemorrhage into the adrenal glands caused by fulminant meningococcemia, adrenal insufficiency, and disseminated intravascular coagulation.[3]
Treatment
Persons with confirmed N. meningitidis infection should be hospitalized immediately for treatment with antibiotics. Because meningococcal disease can disseminate very rapidly, a single dose of intramuscular antibiotic is often given at the earliest possible opportunity, even before hospitalization, if disease symptoms look suspicious enough.[3] Third-generation cephalosporin antibiotics (i.e. cefotaxime, ceftriaxone) should be used to treat a suspected or culture-proven meningococcal infection before antibiotic susceptibility results are available.[17] Empirical treatment should also be considered if a lumbar puncture, to collect CSF for laboratory testing, cannot be done within 30 minutes of admission to hospital.[citation needed] Antibiotic treatment may affect the results of microbiology tests, but a diagnosis may be made on the basis of blood-cultures and clinical examination.[citation needed]Prevention
All recent contacts of the infected patient over the 7 days before onset should receive medication to prevent them from contracting the infection. This especially includes young children and their child caregivers or nursery-school contacts, as well as anyone who had direct exposure to the patient through kissing, sharing utensils, or medical interventions such as mouth-to-mouth resuscitation. Anyone who frequently ate, slept or stayed at the patient's home during the 7 days before the onset of symptom, or those who sat beside the patient on an airplane flight or classroom for 8 hours or longer, should also receive chemoprophylaxis. The agent of choice is usually oral rifampicin for a few days.[3]Vaccination
Main article: Meningococcal vaccine
United States
As of 2008 three vaccines have been available in the U.S. to prevent meningococcal disease for people aged 2 or older. All three vaccines are effective against the same serogroups: A, C, Y, and W-135. Two meningococcal conjugate vaccines (MCV4) are licensed for use in the U.S. The first conjugate vaccine was licensed in 2005, the second in 2010. Conjugate vaccines are the preferred vaccine for people 2 through 55 years of age. It is indicated in those with impaired immunity, such as nephrotic syndrome or splenectomy.A meningococcal polysaccharide vaccine (MPSV4) has been available since the 1970s and is the only meningococcal vaccine licensed for people older than 55. MPSV4 may be used in people 2–55 years old if the MCV4 vaccines are not available or contraindicated. the Centers for Disease Control and Prevention (CDC) publishes information about who should receive meningococcal vaccine.[18]In June 2012, the U.S. Food and Drug Administration (FDA) approved a combination vaccine against two types of meningococcal diseases and Hib disease for infants and children 6 weeks to 18 months old. The vaccine, Menhibrix, was designed to prevent disease caused by Neisseria meningitidis serogroups C and Y, and Haemophilus influenzae type b (Hib). It was the first meningococcal vaccine that could be given to infants as young as six weeks old.[19]
In October 2014 the FDA approved the first vaccine effective against serogroup B, named Trumenba, for use in 10 to 25 year old individuals.[20]
Africa
In 2010, the Meningitis Vaccine Project introduced a vaccine called MenAfriVac in the African meningitis belt. It was made by generic drug maker Serum Institute of India and cost 50 U.S. cents per injection. Beginning in Burkina Faso in 2010, it has been given to 215 million people across Benin, Cameroon, Chad, Ivory Coast, Ethiopia, Ghana, Mali, Niger, Mauritania, Nigeria, Senegal, Sudan, Togo and Gambia.[21]As of January 2015 no case of meningococcal meningitis has been observed among vaccinated populations and transmission has stopped in the belt. On January 8, 2015 the World Health Organization authorized MenAfriVac for routine child immunization in Africa.[21]
Genetic transformation
Genetic transformation is the process by which a recipient bacterial cell takes up DNA from a neighboring cell and integrates this DNA into the recipient’s genome by recombination. In N. meningitidis, DNA transformation requires the presence of short DNA sequences (9-10 mers residing in coding regions) of the donor DNA. These sequences are called DNA uptake sequences (DUSs). Specific recognition of these sequences is mediated by a type IV pilin.[22] In N. meningitides DUSs occur at a significantly higher density in genes involved in DNA repair and recombination (as well as in restriction-modification and replication) than in other annotated gene groups. The over-representation of DUS in DNA repair and recombination genes may reflect the benefit of maintaining the integrity of the DNA repair and recombination machinery by preferentially taking up genome maintenance genes, that could replace their damaged counterparts in the recipient cell.[23]N. meningititis colonizes the nasopharyngeal mucosa, which is rich in macrophages. Upon their activation, macrophages produce superoxide (O2¯) and hydrogen peroxide (H2O2). Thus N. meningitides is likely to encounter oxidative stress during its life cycle.[24] Consequently, an important benefit of genetic transformation to N. meningitides may be the maintenance of the recombination and repair machinery of the cell that removes oxidative DNA damages such as those caused by reactive oxygen. This is consistent with the more general idea that transformation benefits bacterial pathogens by facilitating repair of DNA damages produced by the oxidative defenses of the host during infection.[25]



Post a Comment